Report Library
All Reports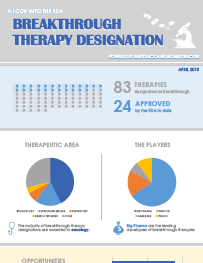
2015 Breakthrough Therapy Designation Infographic - Medtrack / BioMedTracker
April 23, 2015
With the passage of the FDA Safety and Innovation Act of 2012 (FDASIA), the Breakthrough Therapy Designation (BTD) was introduced
as the newest mechanism to facilitate expedited development and review. This infographic summarizes the details of our full report which
provides an overview of the BTD pathway and its evolution over the past three years.
Please download our full report, Breakthrough Therapy Designation: Paving the Way for Innovation, on this page.
This report is a collaborative effort between Medtrack and BioMedTracker. Medtrack’s integrated platform offers insights into pharmaceutical pipelines, sales, epidemiology, patents and more, to give a comprehensive view of the biopharmaceutical business landscape. For more information about Medtrack, please visit Medtrack here.
For our disclosures, please read the BioMedTracker Research Standards.
Please download our full report, Breakthrough Therapy Designation: Paving the Way for Innovation, on this page.
This report is a collaborative effort between Medtrack and BioMedTracker. Medtrack’s integrated platform offers insights into pharmaceutical pipelines, sales, epidemiology, patents and more, to give a comprehensive view of the biopharmaceutical business landscape. For more information about Medtrack, please visit Medtrack here.
For our disclosures, please read the BioMedTracker Research Standards.
| Disease Group Covered: |
Allergy
Cardiovascular Endocrine Hematology Infectious Disease Metabolic Neurology Oncology Ophthalmology Psychiatry Respiratory |
| Indications Covered: |
Castleman's Disease
Dysmenorrhea |
