Report Library
All Reports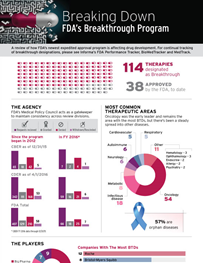
The Pink Sheet Breaking Down FDA's Breakthrough Program - Infographic
April 13, 2016
This infographic provides a review of the impact that the US Food and Drug Administration's expedited approval pathway has had on drug
development and regulatory review to date, using data from Biomedtracker and sister businesses Pink Sheet (FDA Performance Tracker)
and Medtrack.
This infographic is available for free. Visit The Pink Sheet and create a free account to view the full infographic.
For our disclosures, please read the Biomedtracker Research Standards.
This infographic is available for free. Visit The Pink Sheet and create a free account to view the full infographic.
For our disclosures, please read the Biomedtracker Research Standards.
| Disease Group Covered: |
Autoimmune/immunology
Cardiovascular Dermatology Endocrine Infectious Disease Metabolic Neurology Oncology Ophthalmology Psychiatry Respiratory |
| Indications Covered: | Dysmenorrhea |
Additional Resources:
